Five years ago, life changed dramatically for Leonard Narracci. For decades, he taught English five days a week to high school seniors, then clocked in extra hours working weekends and summers at a nearby farm.
“I was always a very active person,”
Narracci says. “But all of a sudden, my energy level started waning. The simplest physical activity—showering—wore me out.”
A battery of tests confirmed that the then 67-year-old was living with cardiomyopathy, or heart failure—a condition affecting about 5 million Americans—which leaves the heart weakened and unable to pump blood efficiently. One clinical tool used to assess heart function is the ejection fraction—a measurement of how well the heart is pumping. Normal ejection fraction is greater than 50 percent. However, if the heart is damaged, it can be significantly lower.
“I had an ejection fraction of 20,” the former teacher says. “My heart was not pumping enough blood or oxygen. I couldn’t do anything and lived like that for four years.”
Narracci was at a crossroad.
“I was in such a state of depression that I felt life just wasn’t worth living anymore,” he recalls. “I told my wife that I had to find a better way to live—if one even existed.”
The road to recovery began on the Internet, where he discovered that scientists were utilizing adult stem cells to treat heart failure, and that this promising approach was under way close to his Florida home. Moreover, the lead researcher—Zannos Grekos, M.D.—was delivering a seminar in nearby Naples. Narracci eagerly attended the presentation, spoke with former patients, then called that same day to schedule an appointment.
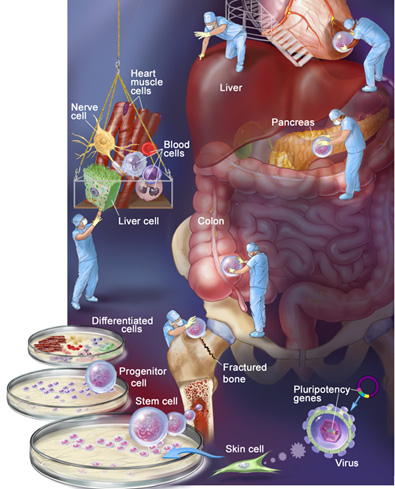
Adult Stem Cells: A Primer
More than a decade ago, Dr. James Thomson, director of regenerative biology at the Morgridge Institute for Research of the University of Wisconsin, derived the first stem cells from human embryos. At the time, scientists said the cells could be induced to turn into any type of tissue in the human body, and the medical world was abuzz with talk of a “human spare-parts kit.” Futurists predicted that repairing hearts and regenerating withered brain regions would be as easy as snapping LEGO bricks into place.
But because the extraction of those cells destroyed the embryos, an ethical and political firestorm arose. Since then, researchers like Dr. Grekos have worked to develop regenerative therapies from adult stem cells harvested from mature human tissue—no embryo required. What’s more, patients can often be treated with their own adult stem cells so there is little danger that their immune systems will reject the cells. If adult stem cells live up to their potential, that vision of snapping new body parts into place might not be so farfetched after all.
There’s a lot of high-profile back-and-forth between proponents of different types of stem cells. But where do various lines of stem cells come from, and what distinguishes them from one another?
Embryonic stem cells. Regenerative science’s basic building blocks, embryonic stem cells are extracted from a cluster of cells which gives rise to an embryo. Some think the cells will revive dying brain cells, repair damaged heart tissue, and mend damaged spinal cords. Critics say mining a developing embryo for stem cells is equivalent to murder.
Cord blood stem cells. Originally touted as a way to bypass ethical concerns associated with embryonic stem cells, stem cells extracted from babies’ umbilical cords haven’t panned out as researchers hoped. In part, that’s because the cells can’t be used in as many therapies as other types. But cord blood stem cell transplants can be a welcome alternative when doctors can’t find a bone marrow donor match for patients who suffer from leukemia.
Induced pluripotent stem (iPS) cells. Though not naturally occurring stem cells, these cells are proof that time travel is possible—at least in a biological sense. Here, scientists train regular adult cells to behave like stem cells. The recipe: Take a culture of normal adult cells (skin or blood cells, for example) and use a modified virus to insert one of several pluripotency genes that are active in embryonic stem cells, turning the developmental clock back to zero. When these modified adult cells are cultured, they can—like embryonic and adult stem cells—turn into other kinds of tissue, including cardiac cells.
Nonembryonic stem cells (adult stem cells). Our body produces these cells for use in everyday processes—fracture healing, tissue regeneration, and new skin growth. Researchers are finding ways to harvest these cells from patients, multiply them in culture, and use them to induce targeted regrowth of failing body tissues.
It was Leonard Narracci’s hope that research in this area could help his weakened heart.
Renewed Hope
In November 2008, Narracci met Dr. Grekos, associate clinical professor at Nova Southeastern University and director of the cardiology and vascular disease branch at Regenocyte—an independent biotechnology firm exploring the potential of adult stem cells. By treating the patient’s stem cells with specific growth factors that the body already uses, the research team was creating a new cell population “educated” to target the damaged area.
“In the past 10 years, we realized that the body’s stem cells possess the ability to regenerate damaged
tissue,” Dr. Grekos explains. “We’ve applied this technology to patients with heart damage.”
The procedure, which is international in scope, takes about one week. “We do the blood draw,” explains Dr. Grekos, who has performed more than 400 of these procedures. Five days after a blood draw, the transplant team reintroduces the stem cells back into the patient’s heart.
In December 2008, Dr. Grekos released findings from a small trial on the potential of adult stem cells in heart failure. “Before treatment, the average ejection fraction in participants was roughly 28 percent,” Dr. Grekos says. “After treatment, ejection fraction reached 40 percent or more. I should add that we have found that patients treated in the early course of their disease fare better.”
Some patients may require additional treatment, but to date, no harmful side effects have been noted.
Quality of life also improved.
“Patients now can walk to the beach, go grocery shopping, and play with grandkids,” Dr. Grekos reports. “We also reduced hospitalization due to congestive heart failure in treated patients by more than 80 percent.”
As with any new radically different approach, reaction from professionals in the field is mixed.
“At first, patients sought us out because they had no other options and nothing to lose,” Dr. Grekos says. “More recently, we’re getting referrals from cardiologists and pulmonologists. It’s becoming more mainstream.”
Because the procedure remains experimental, patients must pay out of pocket for the procedure, which can cost about $64,500—and there’s no guarantee of results.
Delivering on the Promise?
While some specialists talk of adult stem cells as a biological and moral holy grail—able to perform regenerative feats without the ethical baggage that drags down embryonic stem cell research—others disagree. They argue that adult stem cells are less “programmable” than embryonic stem cells, for instance—less conducive to being transformed into a variety of different tissue types.
Scientific results, as always, will tell the story. In the next few years, expect to see large-scale human trials of adult stem cell therapies. Those trials will be the ultimate acid tests, determining whether adult stem cells will go down in history as a failed experiment or as the foundation for a golden age of regenerative medicine.
But Leonard Narracci isn’t waiting for the next few years to tell the tale. For him, the future has already begun.
“One week after the procedure, I was absolutely astounded,” he says. “I felt my energy coming back. At my one-year follow up, my ejection fraction was 52 percent. Now, I trim palm trees, do yard work, and exert myself without getting tired. Life is worth living again.”
To learn more about the promise of adult stem cells in treating Crohn’s disease and reconstructive surgeries, as well as Dr. Grekos’ research, visit saturdayeveningpost.com/stemcell.
News Worth Knowing
With so much adult stem cell research under way, it can be tough to keep track of it all. We’ve pinpointed some breakthroughs scientists have achieved—and explained why their research is worth keeping an eye on.
Type 1 Diabetes: Going Insulin-Free
A diagnosis of type 1 diabetes means investing hours each day injecting insulin and monitoring blood sugar levels. In 2009, however, researchers at Northwestern University and Brazil’s University of São Paulo reported successfully using patients’ adult stem cells to stop the body attacking islet cells of the pancreas. “It’s the first intervention that has ever resulted in patients being completely drug-free,” says study co-author Dr. Richard Burt, chief of the division of immunotherapy at Northwestern University’s Feinberg School of Medicine.
To restore pancreatic function, researchers extracted stem cells from each patient’s bone marrow. After treating the patients with radiation to lower immune resistance, technicians injected the reserved stem cells in such a way that they migrated to the bone marrow and reconstructed the immune system, which enabled the ravaged islet tissue to grow once more. On average, treated patients lived without insulin injections for 31 months.
Bones: Mending Tough Breaks
Most people who break a bone assume they’ll be on the mend within weeks. But 10 to 20 percent of fractures never heal. Anna Spagnoli, an endocrinologist at the University of North Carolina and colleagues deviseda novel way to heal these bone fractures: seeding them with adult stem cells. In 2008 Dr. Spagnoli tested the technique in the lab. She removed adult stem cells from mouse bone marrow, modified them so they would express a protein called insulin growth factor 1 (IGF-1), then transplanted them into other mice with fractured leg bones. Adult stem cells were marked with a fluorescence gene, so she could see that the cells migrated directly to the site of the injury to help heal the break. “The stem cells make more new bone and new cartilage,” she says. She hopes the therapy will enter clinical trials within the next one or two years.
Become a Saturday Evening Post member and enjoy unlimited access. Subscribe now