Your Weekly Checkup: A New Vaccine for Shingles
We are pleased to bring you “Your Weekly Checkup,” a regular online column by Dr. Douglas Zipes, an internationally acclaimed cardiologist, professor, author, inventor, and authority on pacing and electrophysiology. Dr. Zipes is also a contributor to The Saturday Evening Post print magazine. Subscribe to receive thoughtful articles, new fiction, health and wellness advice, and gems from our archive.
Many readers have had chickenpox as a child. I know I did. A virus called varicella-zoster triggers the itchy blisters, along with fever, fatigue, and headache, normally lasting 5-7 days. After causing chicken pox in the child, this crafty virus lies waiting in the nerve tissues of the body, ready to be reactivated for an encore as shingles in the adult. Now the stage is set for potentially more dire consequences, affecting almost one in three U.S. adults.
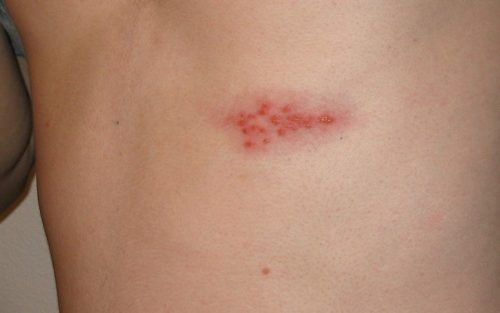
Shingles symptoms range in severity from trivial itching to a very painful rash with nerve damage. In its most severe form, it causes strokes, encephalitis, spinal cord damage and loss of vision. Although shingles can occur anywhere on the body such as the face or near the eye, it most often presents as a single band of blisters that follows a nerve route encircling the left or right side of the trunk. The pain can be minimal, with a bit of itching, to excruciating, requiring narcotics for relief. Shingles most commonly affects people ages 50 or older, those with compromised immune systems such as HIV/AIDS, during cancer treatment or after organ transplant.
Here’s the exciting news: The Food and Drug Administration has just approved a new vaccine called Shingrix manufactured by GlaxoSmithKline for adults ages 50 and older. The FDA’s advisory panel has recommended the Shingrix over Merck’s Zostavax. The latter has been the only shingles vaccine available for more than ten years and was recommended for people ages 60 and older. That’s the one I got several years ago. Now, the Advisory Committee on Immunization Practices is recommending people like me receive the new one, Shingrix. Zostavax was given in one dose, and had shown a 51 percent reduction in shingles and a 67 percent reduction in nerve pain. Shingrix requires two doses, and the company said clinical trials showed it to be about 98 percent effective for one year and about 85 percent over three years. A drawback, however, is that more people had adverse reactions to Shingrix than to Zostavax, including fever and muscle aches. The side effects lasted a few days and were not considered serious.
So, readers, listen to the advice from experts. I am a staunch advocate of the benefits of vaccines and this is one vaccine not to pass up. You do not want to get shingles. Get the vaccine instead. I plan to do just that as soon as it is available commercially—either late in 2017 or January of 2018. Check with your doctor about availability and appropriateness.